Kevlar ®
Kevlar is the registered trademark for a light, strong para-aramid synthetic fiber, related to other aramids such as Nomex® and Technora®.
Developed at DuPont® in 1965 by Stephanie Kwolek and Roberto Berendt, it was first marketed in 1971. Typically it is spun into ropes or fabric sheets that can be used as such or as an ingredient in composite material components.
Toward the end of the 1920s the next important breakthrough for DuPont Corporation came as a result of fundamental rather than applied research. The head of research noted at the time: "We are including in the budget for 1927 an item of $20,000 to cover what may be called, for want of a better name, pure science or fundamental research work...the sort of work we refer to...has the object of establishing or discovering new scientific facts."
In a short time the group that had been put together under this budget had developed an understanding of radical polymerization and established the basic principles for condensation polymerization and the structure of condensation polymers. This led to the invention and commercialization of nylon in 1938, the beginning of the modern materials revolution. (Prior to this, the group invented neoprene synthetic rubber in 1933.)
Many synthetic materials are invented by DuPont research after that, forming the basis for many global businesses and products including household names such as Teflon® fluoropolymer resins and SilverStone® certified non-stick finishes, Stainmaster® flooring systems, Kevlar® brand fiber, Nomex® brand fiber and paper, Lycra® spandex fiber, Sontara® spun-laced fabric, Mylar® polyester film, Tyvek® spunbonded olefin, Cordura® nylon fiber, and Corian® solid surface material.

Currently, Kevlar has many applications, ranging from bicycle tires and racing sails to body armor because of its high strength-to-weight ratio-famously: "...5 times stronger than steel on an equal weight basis..."
A similar fiber called Twaron® with roughly the same chemical structure was introduced by Akzo in 1978, and now manufactured by Teijin.
Kevlar is synthesized from the monomers and terephthaloyl chloride in condensation reaction yielding hydrochloric acid as a byproduct. The result is a liquid-crystalline behavior and mechanical drawing orienting the polymer chains in the fiber's direction.
Some other common synthetic polymers include Nylon, Teflon, Lycra, and polyester. A polymer is a chain made of many similar molecular groups, known as monomers that are bonded together.
It turns out that the orientation of the polymer chains is very important to certain properties of polymers such as flexibility, rigidity, and strength. A group of polymer chains can be organized in a different fiber lines. You can put the polymer chains together randomly in a pile or you can orient them neatly side by side in a row.
A Kevlar fiber is an array of molecules oriented parallel to each other like a package of uncooked spaghetti. This orderly, untangled arrangement of molecules is described as a crystalline structure. Crystallinity is obtained by a manufacturing process known as spinning, which involves extruding the molten polymer solution through small holes. The crystallinity of the Kevlar polymer strands contributes significantly to its unique strength and rigidity
The individual polymer chains are actually held together by electrostatic forces between molecules known as hydrogen bonds.
When it comes to hydrogen bonds, Kevlar and water have something in common. In both compounds, the oxygen atoms have a high density of electrons around the nucleus. Since electrons are negatively charged, this gives the oxygen atoms a slight negative charge. On the other hand, hydrogen atoms have a much lower density of electrons around the nucleus, giving the hydrogen atoms a partial positive charge. Like the north and south poles of magnets, the positive hydrogen and negative oxygen of different molecules attract each other, forming hydrogen bonds.
Scientists can use a special type of x-ray microscopy called XANES to reveal the orientation of molecules in materials. At the National Synchrotron Light Source in New York, scientists exposed the cut end of a Kevlar fiber to produce this image:
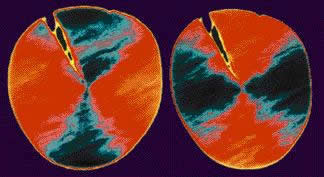
The pattern shows that the aromatic components of Kevlar have a radial (spoke-like) orientation.
The radial orientation is important because it allows the polymer chains to be well-ordered and symmetric like the atoms in a crystal. Because of this highly ordered structure, a fiber of Kevlar has only a few structural flaws or weak places. This lack of flaws or weak places is the biggest reason for the exceptional strength of Kevlar. Kevlar strength is 5 times that of steel and modulus 75% of carbon fiber.
Kevlar (poly paraphenylene terephthalamide) production is expensive because of the difficulties arising from using corrosive concentrated sulfuric acid, needed to keep the water-insoluble polymer in solution during its synthesis and spinning.
Kevlar is the most impact resistant fiber on the market, also exhibits the highest tensile strength and highest resistance to damage, vibration and crack propagation.
However, Kevlar has very low compressive strength, so it is recommended that laminates carrying bending or compression loads have carbon fiber incorporated into the laminate to provide adequate stiffness. Kevlar is also hygroscopic, ultra violet sensitive and un-sandable, thus it is not recommended as a sheathing on outer ply of the laminate. Kevlar is categorized by weight per m2, available in a cloth weave and sold by the running yard.

Kevlar has different types of weaves, and the weave that makes a fabric-like material for vests is called Kevlar 29. Kevlar 29 may also be used in brake lines, or to replace asbestos. It is also is a major part of the composition of body armor.
Kevlar has two other types, Black Kevlar, and Kevlar 49. Black Kevlar may be used to replace rubber items like tires. Kevlar 49 is extremely strong and can replace the more traditional materials used for a boat hull, or be used in simple items like bicycle frames.
On the other hand, it is an excellent material for F1 bodywork, and tyres. As radial cord material, it works well for a couple reasons. It is not a massive part of the entire tyre or necessarily even the cord. Bridgestone, Michelin and Pirelli F1 tires use a multi layered, multi composite cord approach, and Kevlar is used, either pure as one or more layers, or layered and interlayered as a composite as part of an individual layer. layered together and in a tight construction, it holds up pretty well to flex for as long as it needs to, which isn't very long in an F1 tyre. It's also very resistant to high temperatures, and it is usually used in tyre technology as an anti puncture layer, since it is the same material Bullet Proof vests are made from, so it works well. You do have to remember, that some flex is wanted in an F1 tyre, the Kevlar from what I have been told, gives them a better fluxuation in the casing for better variable damping effects from compound to compound.
Some parts of F1 bodywork must be, by the FIA rules covered by Kevlar. For example wings must have final layer made of Kevlar to hold the carbon fiber together in the event of a collision. That will prevent breaking of carbon fiber elements in small pieces. Internal layer of survival cell (monocoque) must be made of Kevlar to prevent penetration of front suspension members inside the tub and hurting driver's legs. This rule was imposed after Michael Schumacher crash in Silverstone, when left front suspension wishbone penetrated monocoque and broke Michael's leg.
In driver helmets, one or more of the layers is made from Kevlar to prevent penetrations in case of incidents.






