 |
NOS Nitrous oxide (laughing gas) |
 |
"I am sure the air in heaven must be this wonder working gas of delight".
So wrote poet Robert Southey of nitrous oxide, N2O, also known as nitrogen oxide, dinitrogen monoxide, hyponitrous acid anhydride and facticious air. However, its most well known name is 'laughing gas' or 'happy gas' due to its intoxicating effects when inhaled. At room temperature, it is a colorless non-flammable gas, with a pleasant, slightly sweet odor and taste.

N2O was first discovered in 1793 by the English scientist and clergyman Joseph Priestley (who was also famous for being the first to isolate other important gases such as oxygen, carbon monoxide, carbon dioxide, ammonia, and sulfur dioxide). Priestley made N2O by heating ammonium nitrate in the presence of iron filings, and then passing the gas that came off (NO) through water to remove toxic by-products.
The reaction he observed was:
2NO + H2O + Fe N2O + Fe(OH)2
After initial trials, Priestley thought that N2O could be used as a preserving agent, but this proved unsuccessful.
Following Priestley's discovery, Humphry Davy of the Pneumatic Institute in Bristol, England, experimented with the physiological properties of the gas, such as its effects upon respiration. He even administered the gas to visitors to the institute, and after watching the amusing effects on people who inhaled it, coined the term 'laughing gas'!
Davy even noted the anesthetic effects of the gas:
"As nitrous oxide in its extensive operation appears capable of destroying physical pain, it may probably be used with advantage during surgical operations in which no great effusion of blood takes place".
However, despite this observation, for the next 40 years or so the primary use of N2O was for recreational enjoyment and public shows. So called nitrous oxide capers took place in traveling medicine shows and carnivals, where the public would pay a small price to inhale a minute's worth of the gas. People would laugh and act silly until the effect of the drug came to its abrupt end, when they would stand about in confusion. Many famous people (of their time) and dignitaries from Clifton and Bristol came to inhale Davy's purified nitrous oxide for recreational purposes.
Nitrous oxide found a more scientific use as an anesthetic in clinical dentistry and medicine in the early 1840s. It is a very safe and popular agent still utilized by dentists today. It is much less toxic than alternatives, such as chloroform, with far less risk of explosion than ether. The main use for N2O is usually as a mild sedative and analgesic. It helps to allay anxiety that many patients may have toward dental treatment, and it offers some degree of painkilling ability. However, when inhaled in pure form in large quantities it will cause death by asphyxiation because at atmospheric temperatures and pressure, the oxygen in nitrous oxide is not available to the body.
At room temperature, N2O is quite unreactive with most substances, including alkali metals, halogens, and even ozone. Nitrous oxide reacts with ozone and is the main naturally occurring regulator of stratospheric ozone. Nitrous oxide is also a major greenhouse gas. Considered over a 100 year period, it has 298 times more impact per unit weight than carbon dioxide. Nitrous oxide makes up an extremely small amount of the atmosphere - it is less than one-thousandth as abundant as carbon dioxide. However, it is 200 to 300 times more effective in trapping heat than carbon dioxide. It has one of the longest atmosphere lifetimes of the greenhouse gases, lasting for up to 150 years.
When heated sufficiently, however, N2O decomposes exothermically to N2 and O2. It is also used as an oxidizer in rocketry (This has the advantages over other oxidizers that it is non-toxic and, due to its stability at room temperature, easy to store and relatively safe to carry on a flight. As a secondary benefit it can be readily decomposed to form breathing air. Its high density and low storage pressure enable it to be highly competitive with stored high-pressure gas systems) and in motor racing to increase the power output of engines. At elevated temperatures, nitrous oxide is a powerful oxidizer similar to oxygen.

What all this have to do with cars!
You have seen it in the movies. Hit a button and in an instant the engine produces 50, 100, 150 or more additional horsepower. Is it magic? Not really.
The explanation is rather simple, but before understanding how a nitrous-oxide system allows an engine to generate a rush of additional horsepower, it's best to understand a little on how the engine itself works.
Internal combustion engines are designed to convert one form of energy to another. The engine takes the energy in the fuel and then, through combustion, the fuel's energy is converted into heat and pressure to produce some power and torque at the flywheel. Even if you can't remember this, all that you need to remember at this moment is that the more fuel that you can combust, the more power that you can make. Bottom line, to make more power we need to be able to combust more fuel.
This brings us to combustion. What do we need to be able to burn more fuel? The answer is simple, as just two things are needed. First, we need a way of adding additional fuel. Second, and more important, we need something that will supply an additional amount of oxygen to let the fuel burn.
In racing, nitrous oxide (often referred as "nitrous" or "NOS" from the acronym NOS which is the brand Nitrous Oxide Systems) allows the engine to burn more fuel and oxygen, resulting in a more powerful combustion.
A property of nitrous oxide is that at about 300 degrees C it breaks down into nitrogen and oxygen.
When it is introduced into the intake of an internal combustion engine, it is sucked into the combustion chamber and, on the compression stroke, when the charge air temperature reaches about 300 °C, a very oxygen-rich mixture results. So the injection of nitrous oxide into an engine means that more oxygen is available during combustion. Because you have more oxygen, you can (MUST) also inject more fuel, allowing the same engine to produce more power. But too much oxygen can become a problem. High levels of oxygen alone will cause detonation and engine damage. To keep things safe, the ratio of air/fuel must be kept in check, so additional fuel must be delivered when the nitrous system is running. To keep enough fuel running into the engine, bigger fuel lines are a must, and a high-output fuel pump is also necessary.
Nitrous oxide has another effect that improves performance even more.
Gas is stored in high pressure tank at about 900 psi. At this pressure, nitrous oxide is in a liquid form. When it is released into an intake manifold at atmospheric pressure, it changes to a gas, expands and vaporizes. When it vaporizes, nitrous oxide provides a significant cooling effect on the intake air.
When you reduce the intake air temperature, you increase the air's density, resulting in a denser charge, further allowing more air/fuel mixture to enter the cylinder. As you add this boost of oxygen, you also get a lower manifold temperature because of the phase change of the nitrous oxide from a liquid to a gas.
When we add extra fuel during nitrous oxide injection, the effect is like a super charger or increasing the compression ratio of the engine. Automotive nitrous systems work like the automotive equivalent of a jet's "afterburner" and is used only for short duration extra bursts of power. Nitrous oxide has this effect because it has a higher percentage of oxygen content than does the air in the atmosphere. Nitrous has 36% oxygen by weight and the atmosphere has 21 to 23%. Additionally, nitrous oxide is 50% more dense than air at the same pressure.
Chemical reaction that happened in the cylinder is as follows:
![]()
If this reaction occurs in the combustion chamber, extra oxygen is produced providing an extra boost. It also has one more benefit. Nitrogen itself buffers the increased cylinder pressure controlling the combustion. For the maximum horsepower and for instant power on demand, there is no substitute for nitrous oxide.

Typical NOS kit for stock car upgrade
Nitrous oxide is also a great value on a dollar-per-unit-power increase when installed and operated properly. The downside, of course, is the fun ends quickly. The power boost lasts as long as the bottle of nitrous. The average bottle is a 5 or 10 kilograms and with a street V6 at 4000 RPM that might be worth 30 to 120 seconds of use.
Although it does provide impressive power gains for naturally aspirated engines, the rate of depletion of nitrous oxide is rapid. It is for this reason, that the use of nitrous oxide is primarily confined to drag racing, street straight racing, and other short duration racing applications. Maximum acceleration is the primary objective for these sports, while endurance is not. Therefore, a car normally carries only a few minutes of nitrous oxide, and the driver uses it very selectively by pushing a button.
Another problem with nitrous oxide system is that it is fairly bulky.
Regardless of who manufacturers the nitrous-oxide system, every nitrous-oxide system accomplishes both tasks of supplying a gas and additional amount of fuel.
 |
 |
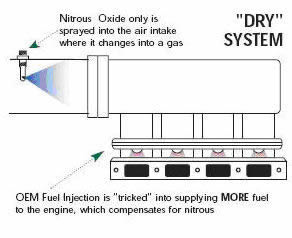
Dry system nozzle |
|
Wet system nozzles |
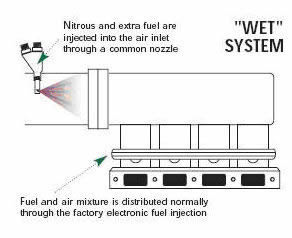
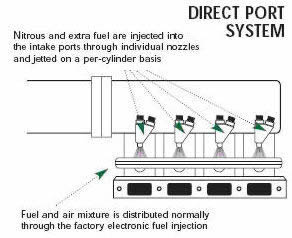
In some way, either through the stock injectors or by adding additional fuel nozzles, the nitrous oxide control system puts more fuel into the engine.
The Basic System
A bottle, high-pressure lines, solenoids, jets and nozzles are included in all nitrous-oxide systems. The nitrous-oxide bottle usually holds 5 to10 kilograms of nitrous oxide and the trunk is the most popular mounting location.
A high-pressure line will carry the nitrous oxide to the solenoid valve. The solenoid is an electronically controlled valve. When a signal of 12 volts is sent to the solenoid, it opens and nitrous oxide is then sent to the nozzle and into the engine. At the same time that nitrous oxide is injected into the engine, the nitrous-oxide system will by some means add additional fuel into the engine. The original and most simplified method of injecting the fuel is by using a solenoid and nozzle setup. This setup is often referred to as a "wet" system and is nearly identical to the configuration for the nitrous except that the fuel is injected at anywhere from 6 to 60 psi, while the nitrous oxide is injected at 700 to 1200 psi.
 |
 |
"Dry" and "Computer Controlled" Systems
The term "dry" nitrous-oxide system refers to nitrous-oxide systems that use the original car injectors to supply the additional fuel into the engine. The original dry systems designed by Nitrous Oxide Systems send a pressure signal to the stock fuel pressure regulator to increase the fuel pressure. This increase in fuel pressure allows the injectors to supply more fuel for the added nitrous oxide. Taking a bit of a different approach, Venom's VC-2000 system uses a car computer control module to increase the amount of time that the injectors are open to put more fuel into the engine. Additionally, the Venom system uses the signal from the vehicle's oxygen sensor to ensure that a safe air-fuel ratio is maintained. If the sensor detects an excess of nitrous without enough fuel, the Venom system shuts off the delivery of nitrous oxide, saving the engine from harm.
High pressure braided hose lines
The Good, the Bad, the Ugly
The good news is that dollar for dollar, the horsepower increase from a properly installed nitrous-oxide system is hard to beat. Most starter systems come ready to generate an additional 40 to 120 hp. Above this power level, a more sophisticated system is required and previous experience with nitrous oxide is highly suggested.
The bad news is that an improperly installed nitrous-oxide system can cause severe engine damage. If nitrous oxide is injected into the engine without supplying an adequate amount of fuel or no fuel at all, then you've got trouble. The temperatures in the combustion chamber will skyrocket, and the engine may detonate and parts will be broken and melted.
There are some ugly facts that you'll have to face when it comes to using a nitrous-oxide system.
- First, bottles are only so big. The more frequently you use the juice, the more times you will be refilling the bottle.
- Second, the less you learn about nitrous, the more likely you are to have a bad experience.
- Third, and most important, it is not the use of nitrous oxide that causes engine damage. It is the misuse of nitrous oxide by the tuner and driver. Every engine has its limit. A stock engine doesn't have the toughness of a racing engine. Chances are that there's a tuner out there with your same engine and they have experience with using nitrous oxide on your engine. If they tell you that a 40-hp shot is all that you can do on a stock engine, believe them.
 NOS solenoid valves
NOS solenoid valves
Better Safe, Than Sorry
If you want to have a good nitrous-oxide system experience there are some considerations you can make to increase the chances of coming away with a smile. Since a nitrous-oxide system relies on the fuel system, it is always best to be sure that your fuel system is at its peak efficiency. Factory fuel filters begin to degrade in performance as early as 10,000 kilometers.
When installing a new nitrous-oxide system, it is recommended that you replace your factory fuel filter and be sure that the injectors are clean.
The other area that you need to address is the vehicle's ignition system. A factory ignition system is designed to operate at near factory horsepower levels. A nitrous-oxide system can easily overcome the capabilities of a stock ignition system. Be sure that the spark plugs are new and that the ignition cables are in good condition. The cap and rotor should also be inspected on non-direct-ignition-system (non-DIS) cars. An ignition amplifier, high-performance ignition wires and spark plugs that are one heat range cooler (for applications over 50 hp). In the absence of an ignition amplifier, it's a good idea to tighten your spark plug gap by 0.25 to 0.1 mm. This will make it easier for your ignition to generate a spark even in the high-horsepower range.
P.S: A friend of mine experimented with the use of Nitrous Oxide (laughing gas) during his teenage years.
At the time, he felt certain it was safe to use, while noting with confidence that it is widely used in dentistry, and is generally regarded as harmless. However, as his experimentation increased, along with a corresponding increase in consumption of the gas, he began to experience a disturbing side affect which impaired the clarity of his vision while under the effect of the gas, and to a lesser degree when the gas had worn off.
The discovery of this unwanted side effect prompted him to discontinue the use of nitrous oxide in a recreational sense, after which, he was relieved to find that the residual impairment of his vision subsided within a short period of time. I thought I might mention my friend's experience, in case you might choose to reference it on your web page in an effort to educate those who might similarly be inclined to regard the gas as being harmless in all regards.
Because nitrous oxide is minimally metabolized, it retains its potency when exhaled into the room by the patient and can pose an intoxicating and prolonged-exposure hazard to the clinic staff if the room is poorly ventilated. Where nitrous oxide is administered, a continuous-flow fresh-air ventilation system or nitrous-scavenging system is used to prevent waste gas buildup. Nitrous oxide can be habit-forming because of its short-lived effect.
In the United States, possession of nitrous oxide is legal under federal law and is not subject to DEA purview. It is, however, regulated by the Food and Drug Administration under the Food Drug and Cosmetics Act; prosecution is possible under its "misbranding" clauses, prohibiting the sale or distribution of nitrous oxide for the purpose of human consumption.
In some countries, it is illegal to have nitrous oxide systems plumbed into an engine's intake manifold. These laws are ostensibly used to prevent street racing and meet emission standards.
Nitrous oxide is entirely legal to possess and inhale in the United Kingdom, although supplying it to others to inhale, especially minors, is more likely to end up with a prosecution under the Medicines Act.
In New Zealand, the Ministry of Health has warned that nitrous oxide is a prescription medicine, and its sale or possession without a prescription is an offense under the Medicines Act. of the chemical, though it is implied that only recreational use will be legally targeted.
Because I'm Croatian, I can say that in my country nitrous oxide is mentioned only in lows talking about medical use. Similarly as in the New Zealand this statement would seemingly prohibit all non-medicinal uses.






